Bacteria-Killing Virus Teams Up with Animal Immune Response to Cure Acute Infections
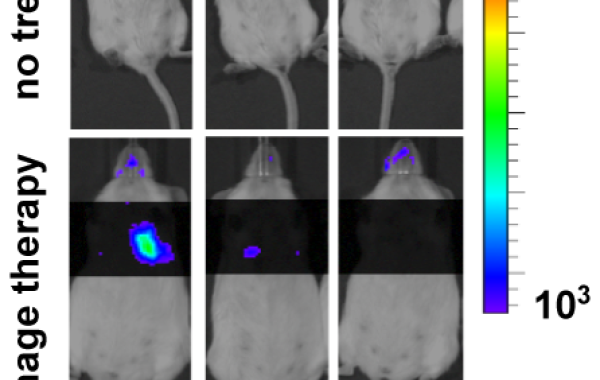
EDITOR'S NOTE: The figure showing the effect of phage therapy on mice was added on July 18, 2017.
The rise of antibiotic-resistant superbugs poses a serious public health threat. In response, scientists and clinicians are exploring alternative ways to cure bacterial infections that are untreatable by antibiotics.
One approach is to use bacteria-killing viruses – also known as bacteriophage, or phage. Phage therapy has been used for nearly a century outside the U.S., most prominently in Russia and Georgia, the former Soviet republic. Clinical trials are ongoing in Europe, including a wound burn treatment trial involving multiple hospitals.
In the U.S., recent successes have heightened interest in moving phage therapy to the clinic. A dramatic example is the cure of a University of California, San Diego, professor who was near death from an infection by the toxin-excreting superbug Acinetobacter baumannii.
Yet, many of the mechanisms underlying phage therapy remain unclear. “The key conceptual challenge is that phage kills individual bacterial cells but not necessarily an entire population of bacteria,” says Joshua S. Weitz, professor of biological sciences and physics at Georgia Tech.
In phage therapy, successful treatment has long been assumed to be due primarily to the phage’s bacteria-killing action. Now, work by Weitz and his team at Georgia Tech and groups led by Laurent Debarbieux and James P. Di Santo at the Institut Pasteur, in Paris, France, finds that immune cells of the animal host act synergistically with phage to cure an otherwise fatal respiratory infection in mice.
“This joint project analyzed the conditions necessary to eliminate pathogen populations when host immune responses are compromised,” Weitz says. The work is published in the July 2017 issue of Cell Host & Microbe.
The researchers investigated the effect of host immunity in an animal model of acute pneumonia caused by multiple-drug-resistant Pseudomonas aeruginosa, a pathogen on the serious-threats list of the Centers for Disease Control & Prevention (CDC). The team – including the paper’s joint first authors, Institut Pasteur’s Dwayne R. Roach and Georgia Tech’s Chung Yin (Joey) Leung – integrated preclinical experimental data with mathematical modeling to characterize interactions between bacteria, phage, and the immune response.
The animal studies indicate that neutrophils – an important type of white blood cell that is part of the body’s major innate defense – are essential to cure the infection during phage treatment. Leveraging results from preclinical experiments and mathematical models, the researchers conclude that neutrophils eliminate what the phage cannot defeat: emerging, phage-resistant P. aeruginosa cells. Together, phage and neutrophils synergistically cure the acute bacterial infection.
The finding has important implications. “In terms of clinical consequences, one could reconsider the selection of patients likely to benefit from phage therapy. It may not be appropriate or recommended for people with severe immunodeficiency,” Debarbieux says. The work will help identify candidates for human phage therapy and could be used to explore synergistic interactions between phage and immune responses in other disease contexts, such as cystic fibrosis.
A second paper describing mathematical details of the therapeutic synergy between phage and the immune system, jointly authored by Leung and Weitz, is published in the Journal of Theoretical Biology.
Work at Georgia Tech was supported by an U.S. Army Research Office grant (W911NF-14-1-0402).
Work at Institut Pasteur was supported by Fondation EDF, Vaincre la Mucoviscidose (IC1011), Association CA.ZO.LA. Luttons contre la mucoviscidose, and the European Respiratory Society (RESPIRE2–2015–8416).




